RSC School Seminar - Assoc Prof Robin Fulton (Victoria University of Wellington)
Title: The “Metallo”-Diels-Alder Reactions: Examining the Metalloid Behavior of Germanimines
Speakers
Event series
Content navigation
Description

The “Metallo”-Diels-Alder Reactions: Examining the Metalloid Behavior of Germanimines
Abstract
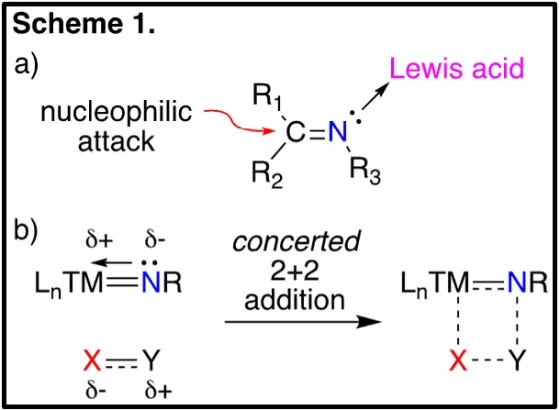
Germanium is commonly classified as a metalloid; that is, its properties are intermediate between that of a metal and a non-metal; however, this classification typically refers to the reduced state of germanium. We are interested in investigating the “metalloid” behaviour of germanium in the higher oxidation states, specifically investigating the chemistry of germanium nitrogen double bonds, or germanimines, LnGe=NR. The chemistry of the carbon-based analogs, or organic imines, is governed by the electrophilic carbon centre and the Lewis basic nitrogen, coordination of the latter to Lewis acids increases the electrophility of the carbon centre, making it more susceptible to nucleophilic attack (Scheme 1a). In contrast, chemistry of the metallic-based analogs, or metal-imido complex, is dominated by concerted [2+2] additions, with electron rich centres adding to the metal and electron poor centres adding to the nitrogen atom (Scheme 1b).
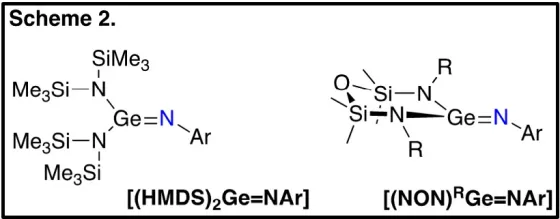
To examine the metalloid behavior of germanimines, we have synthesized two types of germanimine complexes: [(HMDS)2Ge=NAr] and [(NON)RGe=NAr] (Scheme 2), the former containing two monodentate anionic ligands (HMDS) and the latter containing one bidentate dianionic ligand (NONR). The small change in the steric constraints around the germanium results in significant changes to the observed reactivity. [(HMDS)2Ge=NAr] behaves similarly to transition metal imido complexes, undergoing [2+2] cycloaddition reactions with unsaturated substrates. In contrast, [(NON)RGe=NAr] behaves similarly to carbon-based imines, notably undergoing a Diels-Alder reaction with a range of unsaturated compounds.
This chemistry, as well as factors controlling this chemistry, will be discussed.
Location
Building 136, Level 3, STB S1
