Electrical Mechanochemistry
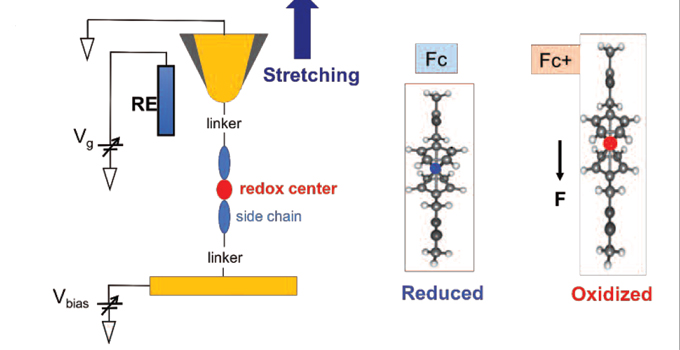
Normally, the redox potential of a molecule is assumed to be a unique property of the molecule, its solvent environment and the temperature. But researchers from the Arizona State University, the Australian National University and Curtin University have now demonstrated that mechanical force can also be used to manipulate electrontransfer reactions (Li Y., Haworth N.L., Xiang L., Ciampi S., Coote M.L., Tao N.J. J. Am. Chem. Soc. 2017, 139, 14 699–706).
Using scanning tunnelling microscopy, the researchers showed that, on stretching, a single molecule of a ferrocene (Cp) derivative undergoes spontaneous oxidation. The oxidation is associated with changes in its singlemolecule conductivity, which could be tracked experimentally.
Calculations confirmed the result and showed that the process is driven by the tendency of the Fe–Cp bond to stretch upon oxidation because of removal of an electron from its bonding orbital.
The observation that mechanical stretching can control the redox states and conductance of a single molecule provides a way to mechanically switch its electronic properties, with potential applications in electromechanical molecular machines.
