Triggering bond cleavage with electric fields
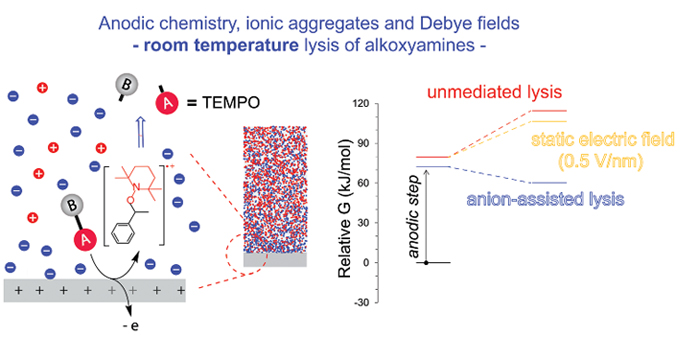
Electricity has long been used in chemistry to trigger electrochemical reactions, but only recently have static electric fields been shown to catalyse non-electrochemical reactions. But implementation has to date required scanning tunnelling microscopy (STM) to orient the reagents appropriately in the electric field. Now, a team of researchers from Curtin University, the Australian National University, the University of Wollongong, ANSTO, the Silesian University of Technology, Poland, and the University of Murcia, Spain, has shown that electrostatic factors contribute to the catalysis of a chemical process that follows an anodic reaction in an electrochemical cell (Zhang L., Laborda E., Darwish N., Noble B.B., Tyrell J.H., Pluczyk S., Le Brun A.P., Wallace G.G., Gonzalez J., Coote M.L., Ciampi S. J. Am. Chem. Soc. 2018, 140, 766–74).
Using STM, the researchers first showed that thermally stable alkoxyamines underwent room-temperature homolysis when exposed to an appropriately aligned electric field. Then, they showed that cleavage also occurred at an electrified electrode–electrolyte interface, albeit with an important twist. The alkoxyamines first underwent one electron oxidation prior to exceedingly fast cleavage into carbocations and nitroxides, the latter driven by the presence of an external static field.
Electrochemical cleavage was shown to proceed for free alkoxyamines in solution, and for alkoxyamines tethered to a silicon electrode, the latter providing a strategy for in situ generation of surface-tethered nitroxides or surface-tethered carbocations.
