RSC School Seminar - Professor Debabrata Maiti (IIT Bombay)
Title: En-Lightening CH Functionalization
Speakers
Event series
Content navigation
Description

En-Lightening C-H functionalization
Abstract
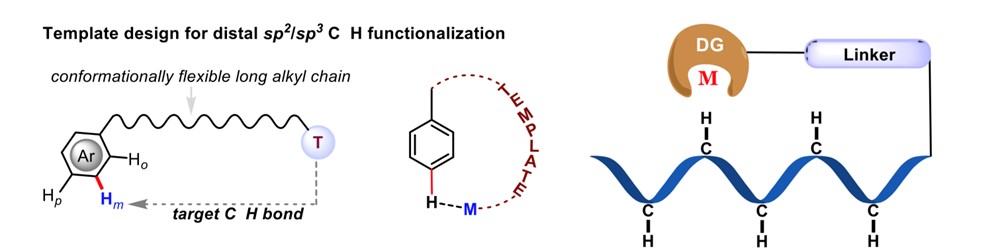
The scientific community has long sought to emulate nature's mechanisms, particularly in understanding how enzymes achieve chemical transformations with precision. Through extensive research, we have gained a thorough understanding of how enzymes catalyze the functionalization of inert C-H bonds in a regio- and stereoselective manner, utilizing metal-active sites. Taking inspiration from these natural processes, we have successfully developed catalytic methods for the functionalization of carbon–hydrogen (C–H) bonds. The Fujiwara–Moritani reaction is one of such reaction which made significant impact on the development of modern C–H activation methodologies. Despite the traditional approach's widespread applicability in various fields, issues related to reactivity and regioselectivity have limited its effectiveness. To revive this remarkable reaction, it is necessary to establish a mechanistic framework that allows simultaneous control over both reactivity and regioselectivity. The conventional high-temperature conditions required for olefination often lead to undesired multiple functionalizations at different sites.
In our work, we have successfully established a photoredox catalytic system by merging a palladium catalyst with an organo-photocatalyst (PC). This innovative system enables selective oxidative olefination of diverse arenes and heteroarenes in a highly regioselective manner. The utilization of visible light plays a crucial role in driving these "regioresolved" Fujiwara–Moritani reactions, eliminating the need for silver salts and high thermal energy. Our catalytic system also exhibits compatibility with both proximal and distal olefination, facilitated by the appropriate directing groups (DGs). This versatility allows us to engage the entire spectrum of C(sp2)–H olefination. The broad scope of this protocol allows for the synthesis of diverse compounds, including natural products, chiral molecules, and drugs. The established mechanistic insights further enhance our understanding of this reaction, enabling future advancements in this field. Importantly, this method offer significant advantages over traditional synthetic approaches, both in terms of economic feasibility and environmental impact.
Recent Refernces:
J. Am. Chem. Soc. 2022, 144, 4; Science, 2021, 372, 701; Nat. Commun. 2021, 12, 1393; Angew. Chem. Int. Ed. 2021, 60, 14030; J. Am. Chem. Soc., 2020, 142, 12453; J. Am. Chem. Soc., 2020, 142, 3762
Presenter Details:
Prof. Debabrata Maiti received his PhD from Johns Hopkins University in 2008 under the supervision of Prof. Kenneth D. Karlin. After postdoctoral studies at MIT with Prof. Stephen L. Buchwald, he joined the Department of Chemistry at IIT Bombay in 2011. His research interests are focused on the development of new and sustainable synthetic and catalytic methodologies. Currently he is an Associate Editor of Journal of Organic Chemistry.
Location
RSC Seminar Room, 3.105, Building 138


