Big and Small Chains of Carbon
We are studying compounds in which a single atom of carbon is held between two metal centres LnM=C=MLn. In most cases the M=C=M spine is linear but we have recently isolated the first examples where the carbon is bent and displays nucleophilic character.
Groups
Project status
Content navigation
About
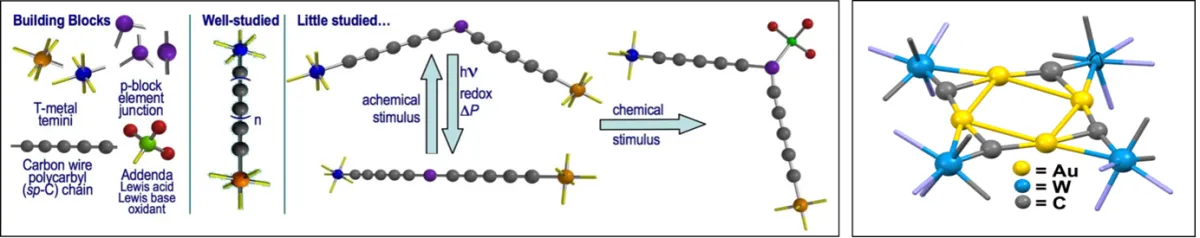
A hypothetical linear chain of carbon atoms “carbyne = C ” is predicted to be the strongest one-dimensional element and expected to show unparalleled thermal and electronic conductivity – It has, however, never been isolated. We have an interest in synthesising smaller linear chains of carbon which have metals at either end, LnMCxMLn(x = 1-6,8,10) as models for molecular scale electronics. One challenge involves installing main-group elements (B, Si, Se, P etc.,) within the chain to moderate the reactivity and physico-chemical properties. At the other end of the scale, we are studying compounds in which a single atom of carbon is held between two metal centres LnM=C=MLn. In most cases the M=C=M spine is linear but we have recently isolated the first examples where the carbon is bent and displays nucleophilic character.