Non-classical Pincer Ligands for Catalytic Applications
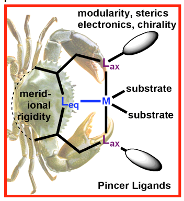
We are developing new pincer frameworks that include unconventional donors, e.g., o-silyl (–SiR3), carbene and o-boryl (-BR2) groups in the equatorial position.
Groups
Project status
Content navigation
About
“Pincer” ligands involve three meridional donors and have proven to be highly effective scaffolds for supporting homogenous catalytic processes. The vast majority are however based on classical donors, e.g., phosphines, amines etc. We are developing new pincer frameworks that include unconventional donors, e.g., o-silyl (–SiR3), carbene and o-boryl (-BR2) groups in the equatorial position. Boron and silicon are exceedingly electropositive elements and exert a superlative trans influence activating substrates. More unusual, however, is the ability of hydride ligands to migrate between these elements and the metal center and in doing so, the polarity of the hydrogen switches from M–H+to Si–H–or B–H–.This polarity reversal is potentially useful in mediating hydrogen transfer/storage for the catalytic activation of small molecules (CO2, N2, SO2, HCCH), e.g., a catalyst that methylates amines (R-NH2 → R–NMe2) under mild conditions with only CO2 as the carbon source.