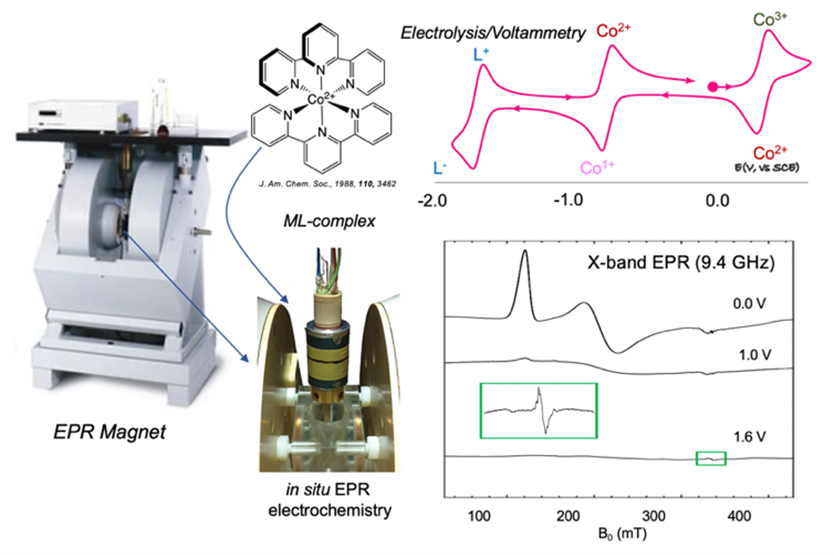
Redox non-innocent first row transition metal complexes - collaboration with Prof. Sally Booker (Otago University, New Zealand)
EPR and related double resonance techniques allow us to elucidate changes in the localization of electron density (metal or ligand centred) following reduction or oxidation, and thus predict likely routes of chemical reactions/catalysis.
Groups
Research themes
Project status
Content navigation
About
Metal complexes possessing redox active ligands open up vast new applications in the chemical and materials sciences, particularly in catalysis. The ability to control and tune metal-ligand reactivity is fundamental to expanding the current scope of catalytic processes, and developing more selective and efficient synthetic pathways to ensure a sustainable society. We use electrochemistry combined with Electron Paramagnetic Resonance (EPR) to study the properties of such complexes. EPR is the paramagnetic analogue of NMR - we detect unpaired electron spins to characterize the three-dimensional and electronic structure of a molecule. EPR and related double resonance techniques allow us to elucidate changes in the localization of electron density (metal or ligand centred) following reduction or oxidation, and thus predict likely routes of chemical reactions/catalysis.