Water harvesting materials
This project investigates thermoresponsive polymer desiccants for sustainable atmospheric water harvesting. By exploring structure-property relationships, it aims to design efficient, energy-saving materials that enable liquid-phase water recovery and reduce industrial environmental impact.
Groups
Research themes
Project status
Content navigation
About
Water scarcity is one of the global issues at the forefront of societal consciousness. A proposed partial solution has been to harvest atmospheric water, a ubiquitous yet underutilised source of water available even in arid areas. Dehumidification is an energy-intensive process that is vital to many industries. Making this process more sustainable would reduce the environmental impact of manufacturing. Hygroscopic polymer desiccants have the potential to alleviate both issues. The general working mechanism of a polymer desiccant (Figure 1) shows cyclical water capture and release steps. Compared to desiccants like zeolites or MOFs, thermoresponsive polymers allow for water to be recovered in the liquid phase rather than the gaseous phase. This removes the need for condensers and allows for more energy-efficient desiccant renewal. This project will explore the structure-property relationships for this class of polymers using a variety of monomers and synthetic methods. The desirable properties are thermoresponsivity, hygroscopicity, and hydrophilicity. The goal of this work is to gather a more holistic understanding of the molecular design of hygroscopic polymer desiccants and consolidate current developments to synthesise novel desiccants with thermoresponsive properties.
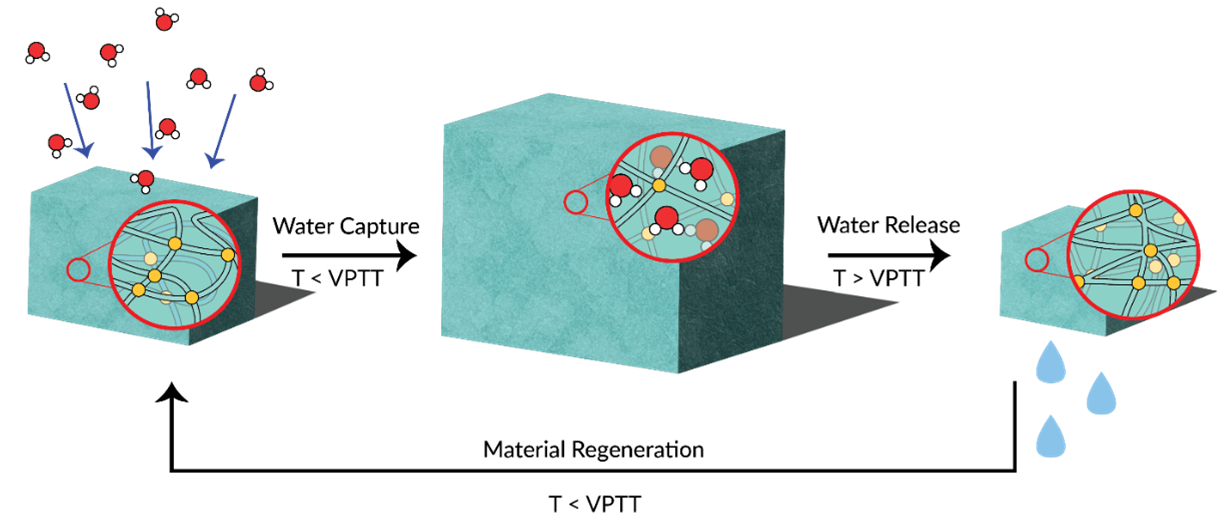
Figure 1: General working mechanism of a polymer desiccant. Starting from the dry xerogel (left), the polymer absorbs atmospheric water (middle) while the temperature is below the volume phase transition temperature (VPTT), leading to saturation (right). The temperature is then set above the polymer’s VPTT, which induces phase separation and therefore release of water in the liquid phase. At this point, the polymer returns to being a xerogel (left).
